
Mitochondrial Biogenesis Review
In the quest for longevity and the mitigation of age-related diseases, sirtuins have emerged as intriguing molecular entities with profound implications for human health and longevity. This review delves into the multifaceted roles of sirtuins, exploring their mechanisms of action, physiological significance, and therapeutic potential.
Introduction
The aging process has long been a subject of fascination and inquiry among scientists and laypeople alike. As humans strive to extend their lifespan and improve the quality of their later years, understanding the underlying biological mechanisms of aging becomes paramount. Sirtuins, a family of highly conserved NAD+ dependent protein deacetylases, have garnered significant attention for their pivotal roles in modulating various cellular processes associated with aging and age-related diseases.
The Sirtuin Family: An Overview
Sirtuins, originally identified in yeast as silent information regulators (Sir2), are evolutionarily conserved proteins found in organisms ranging from bacteria to humans. In mammals, there are seven sirtuin isoforms (SIRT1-7), each localized to different cellular compartments and exhibiting distinct substrate specificity and functions. SIRT1, the most extensively studied member, primarily localizes to the nucleus and plays a central role in orchestrating cellular responses to metabolic stress and environmental cues.
Mechanisms of Action
Sirtuins exert their effects through various molecular mechanisms, with protein deacetylation being the most well-characterized. By removing acetyl groups from lysine residues on histones and non-histone proteins, sirtuins modulate gene expression, protein function, and cellular signaling pathways. Additionally, sirtuins possess ADP-ribosyltransferase activity, allowing them to modify target proteins and regulate diverse cellular processes such as DNA repair, metabolism, and apoptosis.
Physiological Functions of Sirtuins
The pleiotropic effects of sirtuins extend across multiple physiological systems, encompassing metabolism, aging, inflammation, and stress response. In metabolic regulation, SIRT1 acts as a metabolic sensor, integrating nutritional cues and energy status to regulate gluconeogenesis, lipid metabolism, and insulin sensitivity. Moreover, sirtuins play critical roles in modulating mitochondrial biogenesis and function, thereby influencing cellular energy metabolism and oxidative stress resistance.
Beyond metabolic regulation, sirtuins exert profound effects on the aging process and age-related pathologies. Experimental studies have demonstrated that activation of sirtuins can extend lifespan in various model organisms, including yeast, worms, flies, and rodents. Furthermore, sirtuins confer protection against age-related diseases such as neurodegeneration, cardiovascular disease, and cancer through their anti-inflammatory, antioxidant, and pro-survival properties.
Regulation of Sirtuin Activity
The activity of sirtuins is tightly regulated by various endogenous and exogenous factors, including NAD+ availability, post-translational modifications, and environmental stressors. NAD+ serves as an essential cofactor for sirtuin-mediated deacetylation reactions, linking their activity to cellular metabolic status. Consequently, alterations in NAD+ levels, such as those occurring during calorie restriction or in response to pharmacological interventions, can modulate sirtuin activity and exert profound effects on cellular physiology and healthspan.
Therapeutic Implications
Given their diverse roles in cellular homeostasis and disease pathogenesis, sirtuins have emerged as promising therapeutic targets for age-related disorders. Pharmacological agents that activate sirtuins, such as resveratrol, a naturally occurring polyphenol found in red wine, and synthetic small molecule activators, have shown efficacy in preclinical models of aging and age-related diseases. However, translating these findings into clinical practice remains a formidable challenge, necessitating further research into the safety, efficacy, and pharmacokinetics of sirtuin modulators in humans.
Challenges and Future Directions
While the potential of sirtuins as targets for age-related interventions is enticing, several challenges and unanswered questions remain. Clarifying the tissue-specific roles of individual sirtuin isoforms, elucidating their precise molecular targets and downstream signaling pathways, and developing selective modulators with improved pharmacokinetic profiles are critical areas of investigation. Furthermore, the interplay between sirtuins and other longevity pathways, such as insulin/IGF-1 signaling and mTOR signaling, warrants further exploration to uncover synergistic therapeutic strategies for promoting healthy aging.
In conclusion, sirtuins represent a fascinating nexus of molecular biology, metabolism, and aging research, offering tantalizing prospects for extending healthspan and lifespan. Their pleiotropic effects on cellular physiology and their intricate regulatory mechanisms underscore their importance as key players in the aging process and age-related diseases. By unraveling the complexities of sirtuin biology and harnessing their therapeutic potential, we may unlock new avenues for enhancing human health and longevity in the years to come.
Mitochondrial Biogenesis FAQ
What is mitochondrial biogenesis?
Mitochondrial biogenesis is the process by which new mitochondria are formed within a cell. This process involves the growth and division of existing mitochondria and the synthesis of new mitochondrial components, including proteins and lipids.
Why is mitochondrial biogenesis important?
Mitochondrial biogenesis is crucial for maintaining cellular energy production, as mitochondria are the primary site of ATP synthesis. It also helps in maintaining mitochondrial function, adaptability to increased energy demands, and cellular health.
What factors stimulate mitochondrial biogenesis?
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Autem dolore, alias, numquam enim ab voluptate id quam harum ducimus cupiditate similique quisquam et deserunt, recusandae.
How is mitochondrial biogenesis regulated?
Mitochondrial biogenesis is regulated by various signaling pathways and transcription factors.
Key regulators include:
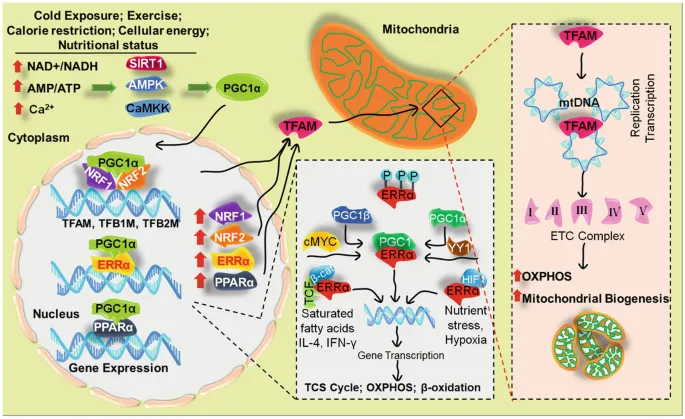
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α): A master regulator that coordinates the expression of genes involved in mitochondrial biogenesis.
Nuclear respiratory factors (NRF1 and NRF2): Transcription factors that regulate mitochondrial DNA (mtDNA) transcription and replication.
Estrogen-related receptor alpha (ERRα): A transcription factor that works with PGC-1α to promote mitochondrial biogenesis.
What role does exercise play in mitochondrial biogenesis?
Regular physical exercise is one of the most effective stimulators of mitochondrial biogenesis. Exercise induces the production of PGC-1α and other factors that promote the growth of new mitochondria and improve mitochondrial function in muscle cells.
How does aging affect mitochondrial biogenesis?
As individuals age, mitochondrial biogenesis tends to decline, leading to a reduction in mitochondrial function and an increase in oxidative stress. This can contribute to age-related diseases and a decrease in overall cellular energy production.
What is the relationship between mitochondrial biogenesis and metabolic disorders?
Impaired mitochondrial biogenesis is associated with various metabolic disorders, including obesity, diabetes, and cardiovascular diseases. Enhancing mitochondrial biogenesis through lifestyle changes or therapeutic interventions can help improve metabolic health.
Can mitochondrial biogenesis be influenced by diet?
Yes, certain dietary components can influence mitochondrial biogenesis. For example, nutrients like omega-3 fatty acids, polyphenols, and certain vitamins (e.g., vitamin B12) have been shown to support mitochondrial biogenesis and function.
Are there any supplements that support mitochondrial biogenesis?
Some supplements, such as resveratrol, curcumin, and certain compounds like alpha-lipoic acid and coenzyme Q10, are believed to support mitochondrial biogenesis and function. However, it's important to consult with a healthcare provider before starting any supplements.
How does mitochondrial biogenesis affect overall health and longevity?
Enhanced mitochondrial biogenesis is associated with improved cellular function, increased energy levels, and better overall health. It may also contribute to increased longevity by reducing the impact of age-related decline and supporting metabolic health.
What research is being done on mitochondrial biogenesis?
Current research on mitochondrial biogenesis focuses on understanding the mechanisms regulating this process, identifying therapeutic targets for metabolic and age-related diseases, and exploring ways to enhance mitochondrial function through lifestyle, diet, and pharmaceuticals.
Can mitochondrial biogenesis be a potential target for disease treatment?
Yes, targeting mitochondrial biogenesis holds promise for treating various diseases, including neurodegenerative disorders, metabolic syndrome, and cardiovascular diseases. Researchers are exploring ways to modulate mitochondrial biogenesis to improve health outcomes and treatment efficacy.
These FAQs cover essential aspects of mitochondrial biogenesis, its regulation, and its implications for health and disease.
Explore the Perfect Health Sciences Website

The information on this site is not intended or implied to be a substitute for professional medical advice, diagnosis or treatment. If you or any other person has a medical concern, you should consult with your health care provider or seek other professional medical treatment immediately. Our products are intended to be refrigerated.
Email us at [email protected]. Our mailing address is #113-14088 Riverport Way, Richmond, BC, V6W 0A7.